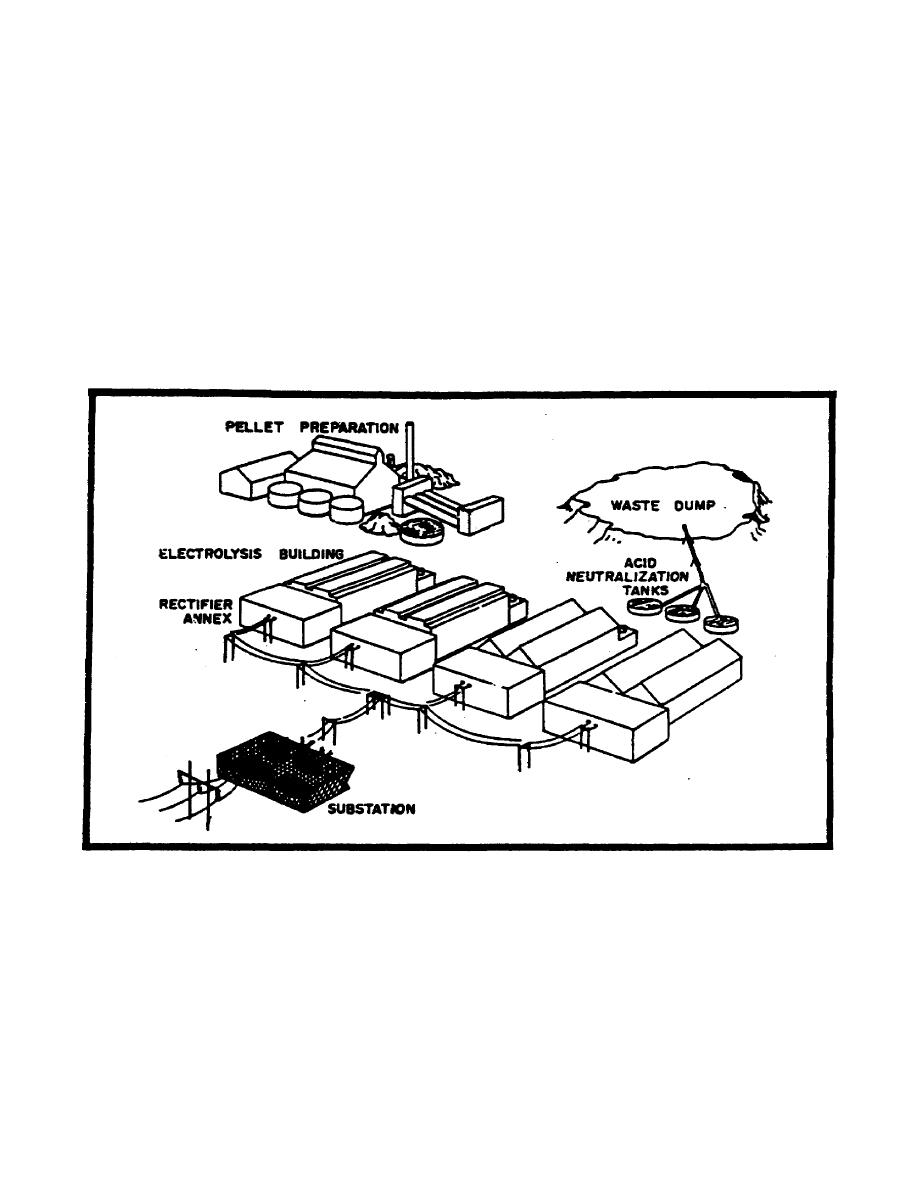
e. Magnesium reduction plants are limited worldwide and small and difficult to
analyze. The process is accomplished by electrolysis (Figure 2-12).
(1) Magnesia is taken to pellet preparation where-it is mixed with various
materials.
The pellets are then melted in the presence of chlorine gas and
magnesium chloride is formed.
This is then, placed in the electrolytic cells in
the presence of hydrogen and heated, allowing the oxygen and chlorine to be freed,
leaving pure magnesium.
The magnesium is normally cast for shipping in the
electrolysis building.
(2) Low voltage direct current is required for the electrolytic process, which
is obtained from conventional sources.
Rectification to direct current will be
accomplished in an annex to the electrolysis building.
(3) Waste material in this process is treated to neutralize the acids and then
dumped.
(4) A chlorine plant may be found in association with magnesium reduction.
Figure 2-12. Ore Reduction Plant (Carbothermic Magnesium).
29
IT 0673



 Previous Page
Previous Page
