A chemical cell is defined as a device that generates electricity by converting chemical energy
to electrical energy.
As chemical energy is converted to electrical energy, by chemical action in a cell, electricity is
produced.
A cell consists of ELECTRODES and ELECTROLYTE. When the electrodes are immersed in
the electrolyte, the chemical action between them generates electricity.
Electrodes are conductors by which electrons leave or return to a cell. The material an
electrode is made of makes it either positive or negative.
Electrolyte is a dilute solution of acid or alkali, and may be either liquid or a moist paste.
Electrolyte is the conductor of electrons from electrode to electrode within the cell.
The chemical action in a PRIMARY CELL usually erodes the negative electrode. As this takes
place, the composition of the electrolyte is changed and it becomes unusable. A primary cell
cannot be recharged. It can be RESTORED by replacing the eroded electrode and the
electrolyte.
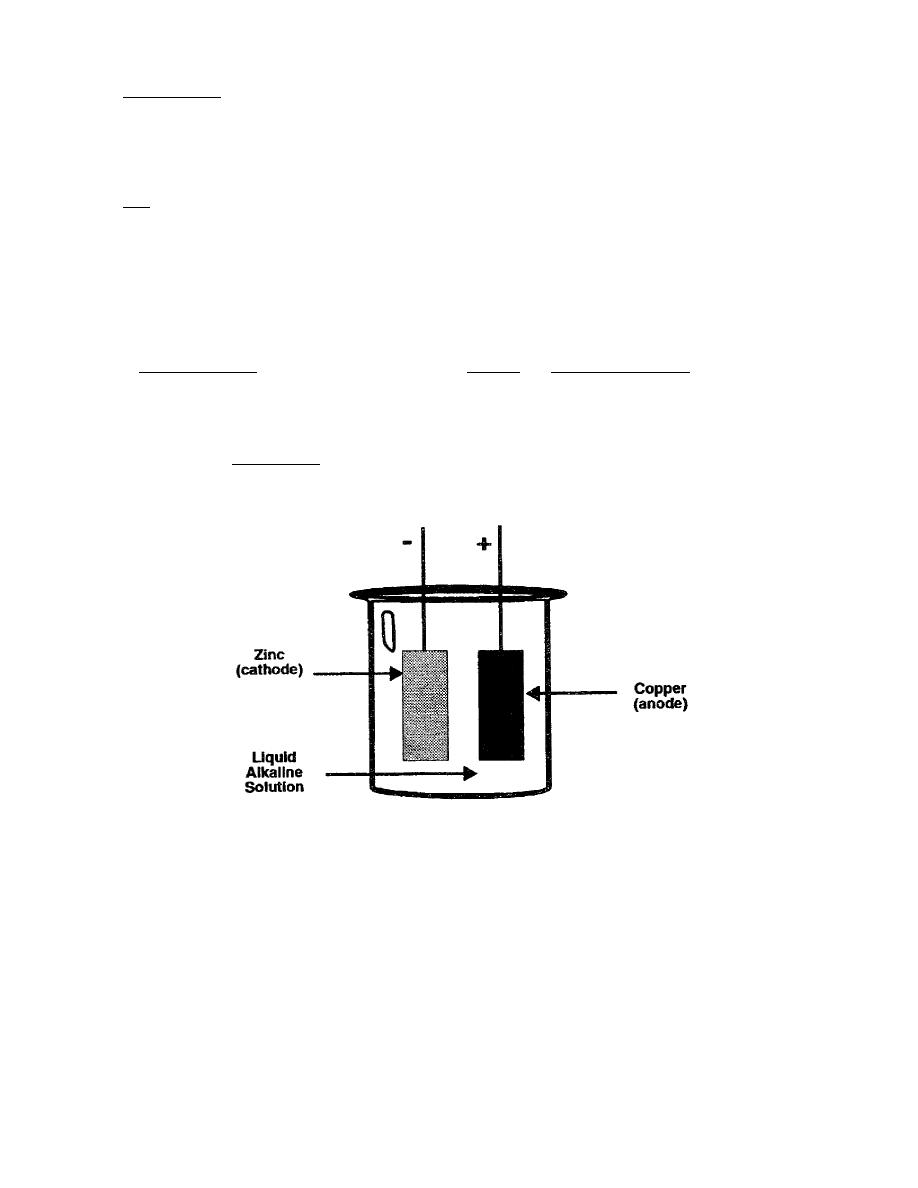
An example of a primary cell is shown below. The electrodes are made of zinc and copper.
The zinc electrode is the negative electrode, or cathode, of the cell. The copper electrode is
the positive electrode, or anode of the cell. The electrolyte of the cell is an alkaline solution.
Figure 1-1. Primary Cell.
IT0335
1-2



 Previous Page
Previous Page
