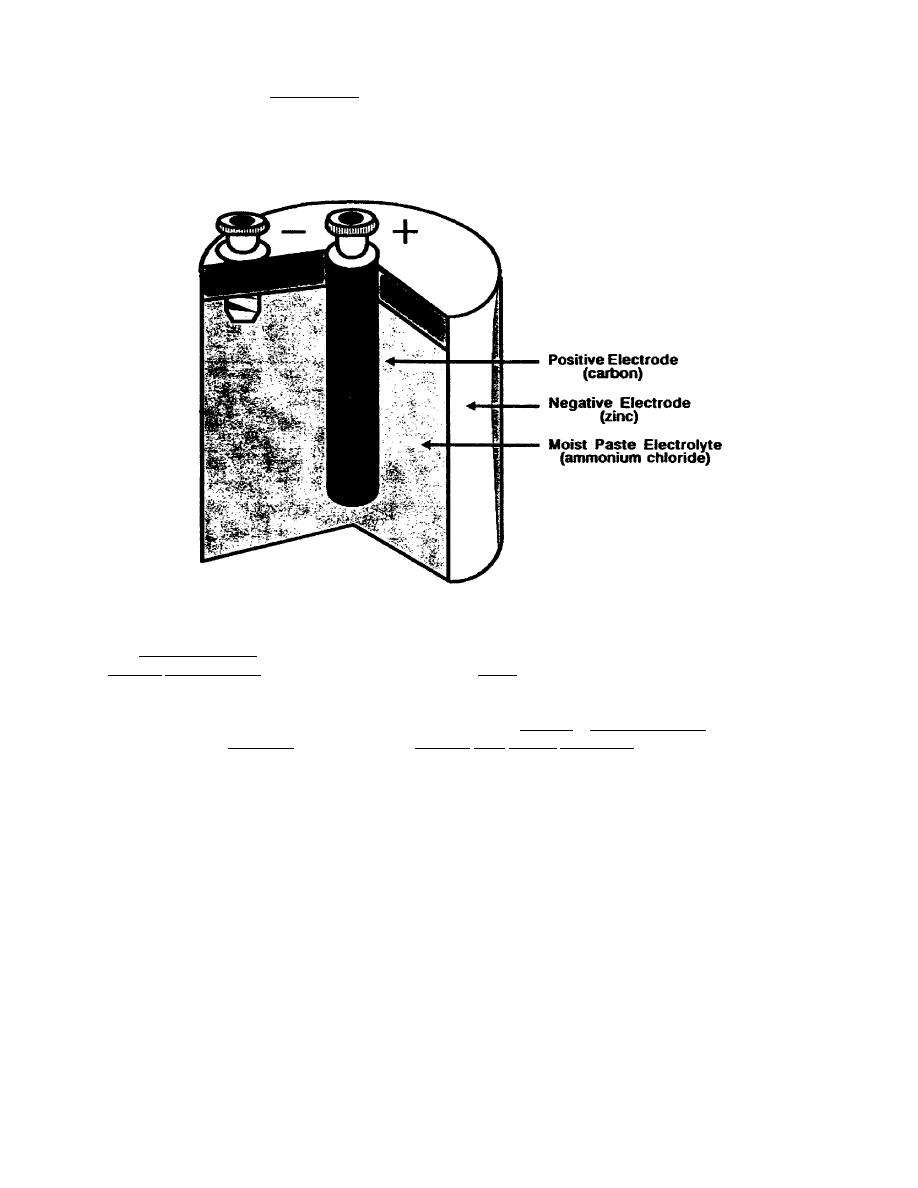
Another example of a primary cell is shown below. This cell has a positive electrode that is
made of carbon and a negative electrode made of zinc. The zinc electrode is also the
container for the other pars of the cell. The electrolyte is a moist paste that contains the
chemicals necessary to generate electricity as a chemical action takes place between it and
the electrodes.
Figure 1-2. Primary Cell.
The chemical action that takes place in a SECONDARY CELL as it generates electricity
causes the materials that make up the electrodes to be transferred from one electrode to the
other.
A secondary cell can be CHARGED or RECHARGED by forcing a current through it in a
direction that is opposite the direction of current flow during discharge.
When a secondary cell is recharged, the materials that were transferred from one electrode to
the other change back to the original materials that were used to make the cell.
1-3
IT0335



 Previous Page
Previous Page
