39. (Continued)
The atomic mass unit (AMU) is equal to ___________ the mass of the
(number)
____________ atom. The sum of the protons and the neutrons of the
nucleus is called ___________ ___________ ___________ .
one-twelfth
40. Most of the elements exist in several isotopic forms, each of which differs in
carbon
atom mass
number
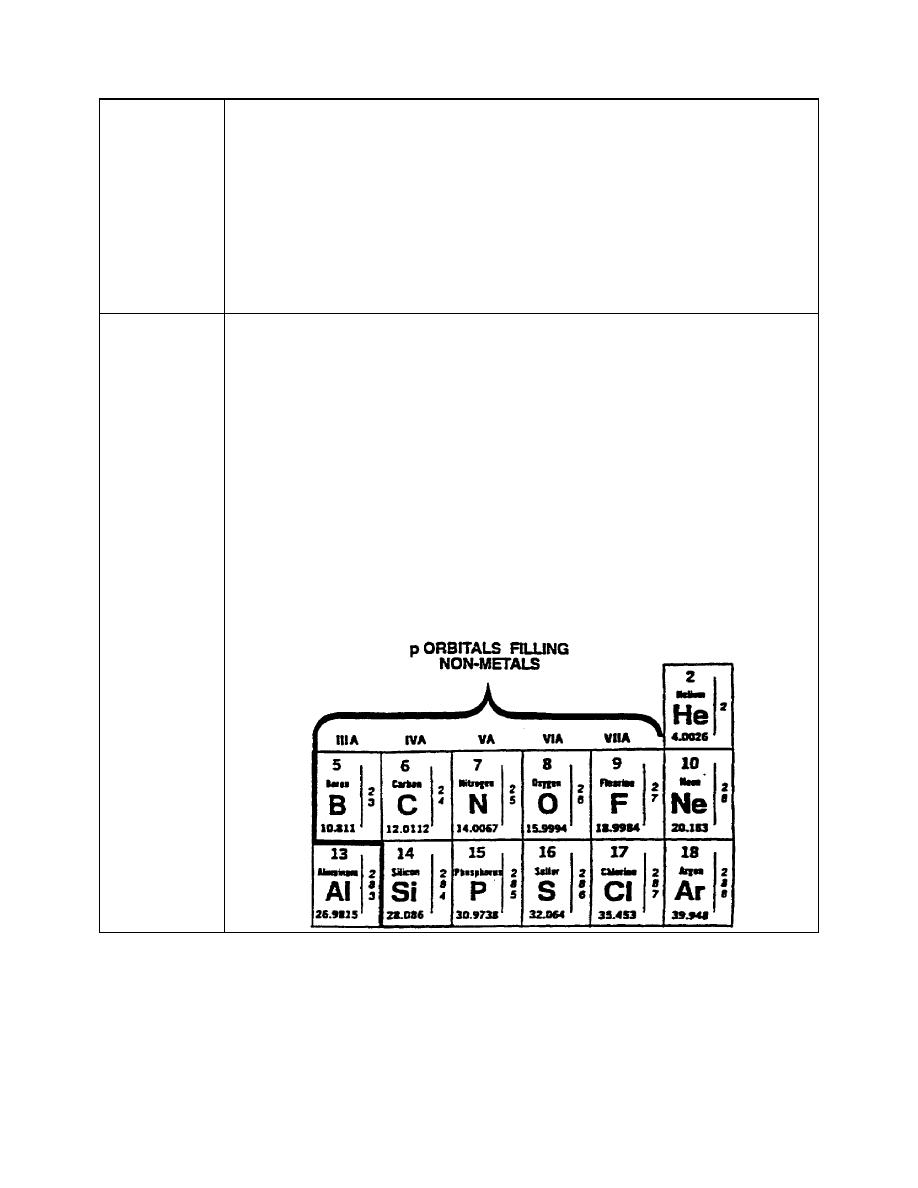
varies with the element considered. Over 98 per cent of the carbon found in
the earth is the C12 isotope. The relative atomic weight of an element is the
average mass of its natural isotopic mixture. There are six isotopes of
carbon, and the average of their masses is called the atomic weight of
carbon. The relative atomic weight of carbon is found on the section of the
periodic chart shown below.
1-29
IT0341



 Previous Page
Previous Page
