(1) b.
2. Much has been learned about the inner structure of the atom in the last
(2) c.
hundred years. So much is stiff theoretical that any attempt to portray the
(3) a.
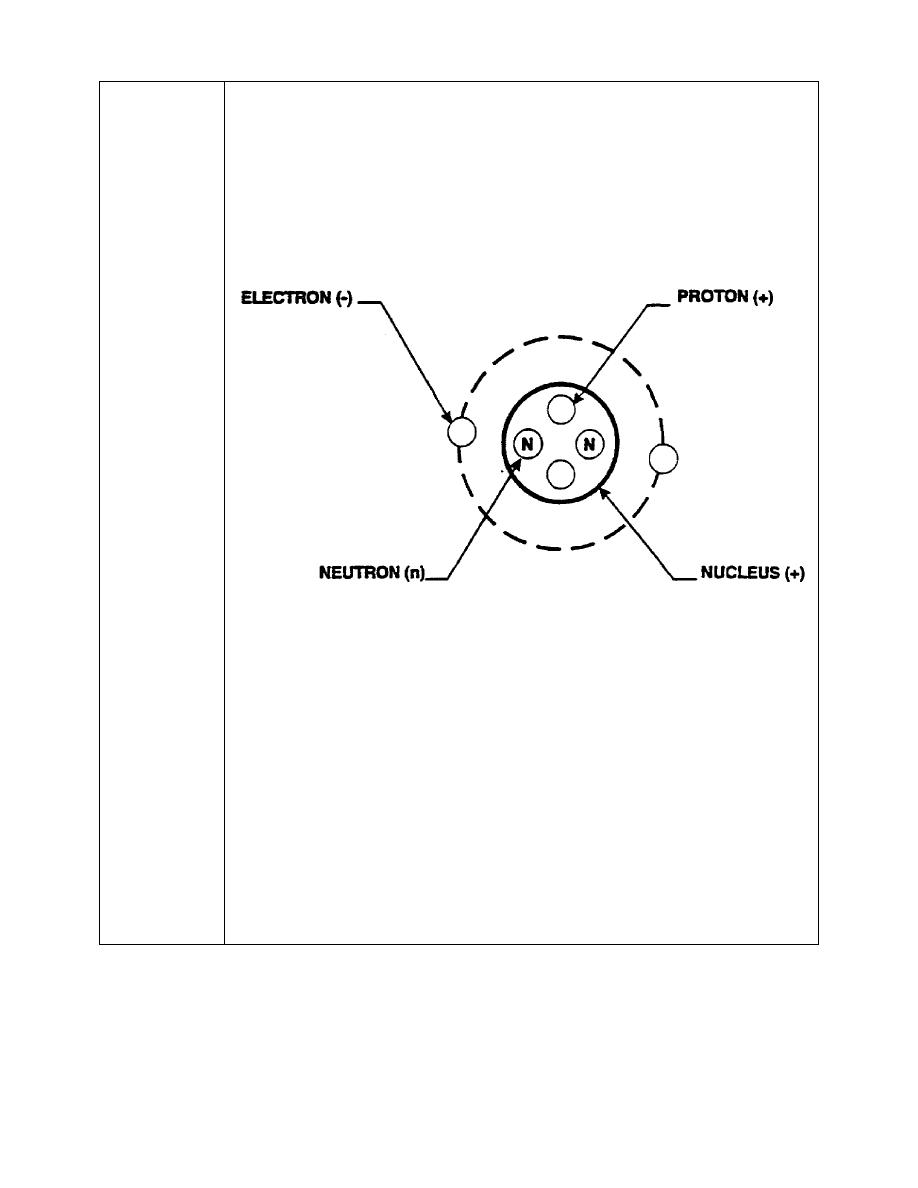
atom pictorially is to some extent a distortion. It is convenient to picture the
atom as an orbital system, as in the figure below.
The NUCLEUS is an extremely small part of the atom, yet most of the mass is
contained therein. The nucleus is made up of positively charged particles
called PROTONS and particles called NEUTRONS with no (neutral) electrical
charge. ELECTRONS, which orbit around the atomic nucleus, are about the
same size as the nucleus but have smaller masses. The electrons are
negatively charged. A neutral atom has the same number of electrons as
protons, so the electrical charges cancel.
1-3
IT0341



 Previous Page
Previous Page
