a. Nucleus (+)
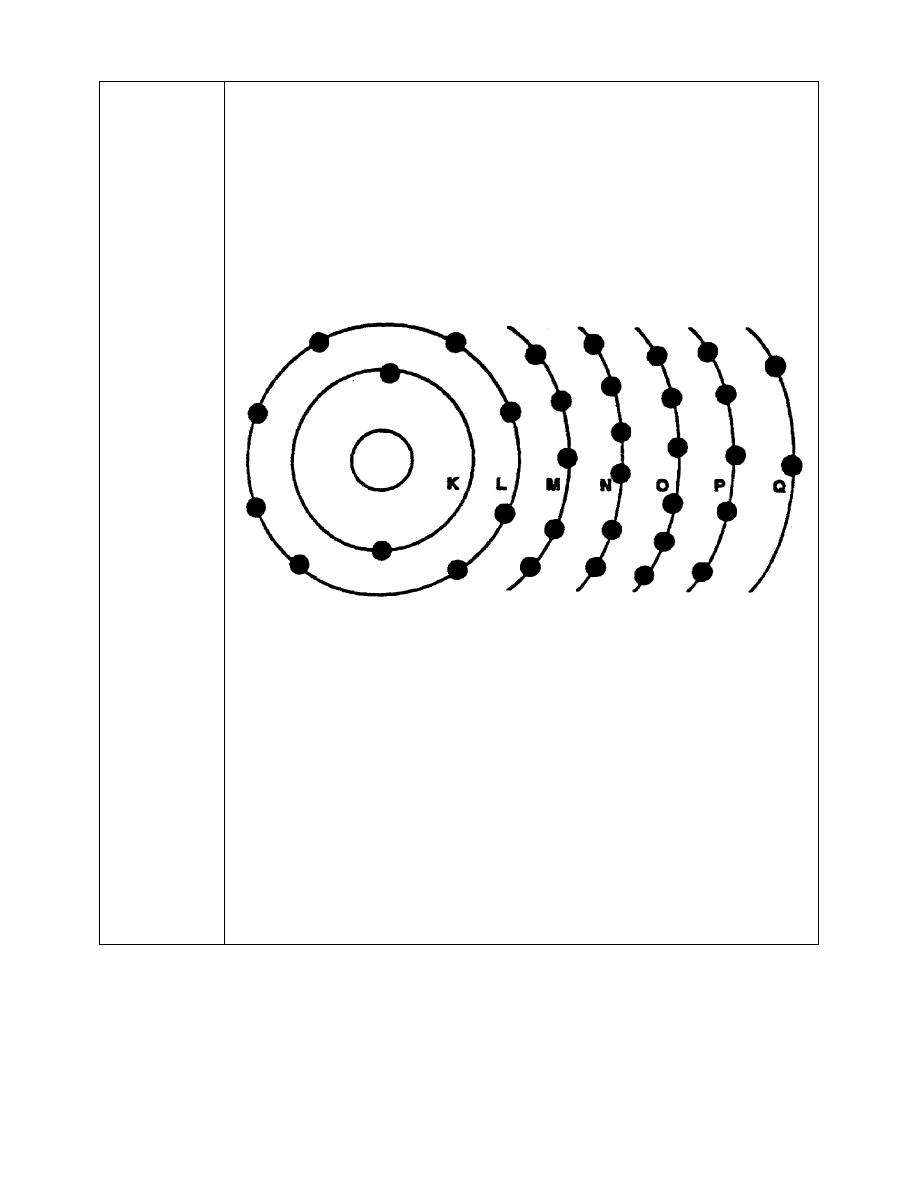
5. The electrons of an atom orbit the nucleus in concentric rings or energy
b. Neutron (n)
levels, and their exact number and arrangement determine how the atom
c. Proton (+)
will combine with atoms of other elements. As illustrated below, the energy
d. Electron (-)
level nearest the nucleus is called the K shell. The remaining shells follow in
alphabetical order up to a maximum of seven energy levels.
The greatest number of electrons that can exist in any level is equal to
two times the shell number squared:
No. = 2 (shell number2)
To determine the maximum number of electrons in the second, or L, shell,
simply square the number of the shell and multiply that value by 2:
No. =2 (22)
IT0341
1-6



 Previous Page
Previous Page
