5. (Continued)
No. = 2(4)
No. =8
Therefore, the maximum number of electrons that can exist in the second, or
L, shell is eight.
Compute the maximum number of electrons that can exist in the fourth,
or N, shell.
No. =
32
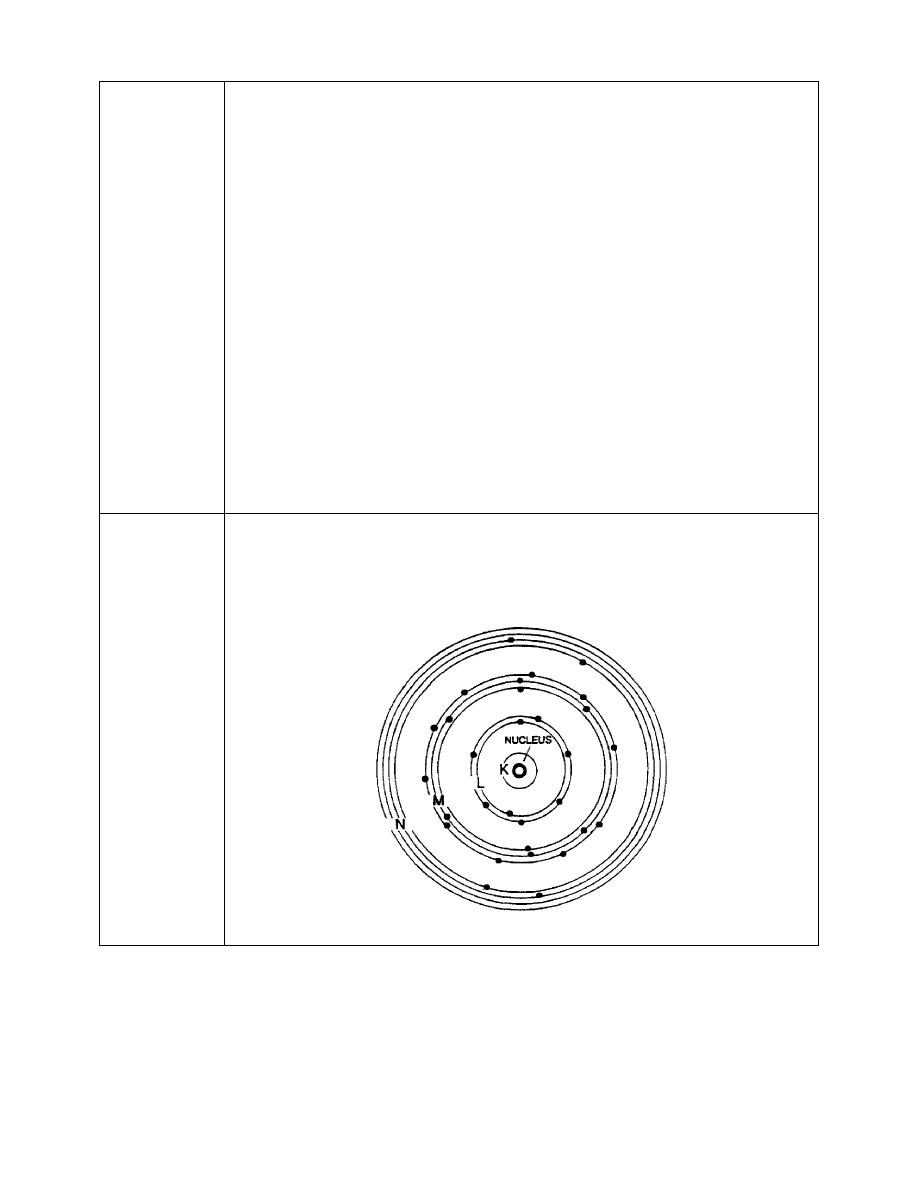
6. The electrons in each main shell around the nucleus of an atom are further
divided into subshells, as illustrated below.
1-7
IT0341



 Previous Page
Previous Page
