maximum
21. There are three types of chemical bonding-ionic, covalent, and metallic-all of
which are closely associated with the outermost electrons of the participating
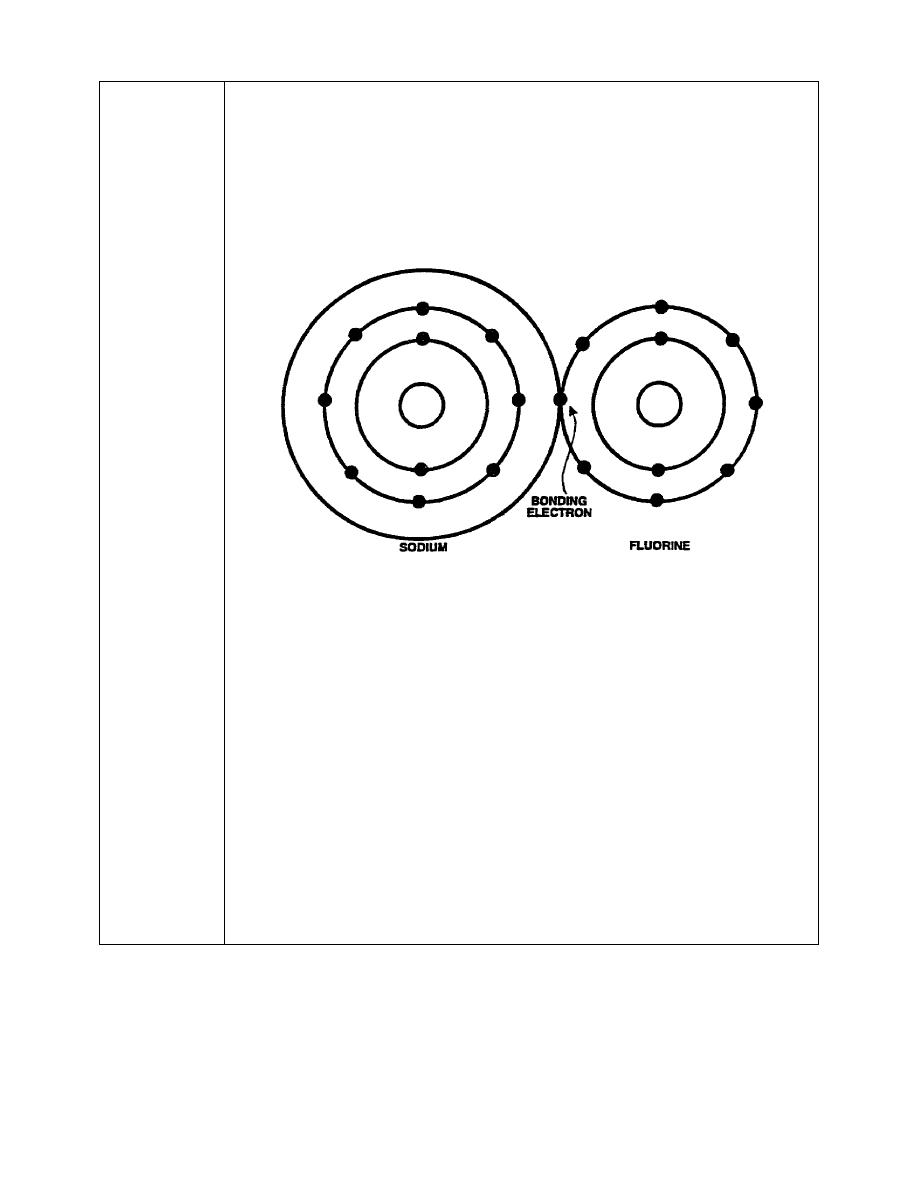
atoms. Ionic bonding occurs when one atom gives up an electron that is
used by the other to achieve chemical stability, as shown below.
When brought into close proximity with fluorine, which contains seven
valence electrons, the sodium atom allows its one valence electron to be
has taken place, both atoms appear to have the eight valence electrons
required for chemical stability. When an atom gives up or takes on additional
electrons, it is called an ion, from which the name "ionic bonding" is derived.
1-17
IT0341



 Previous Page
Previous Page
