14. (Continued)
The elements increase in atomic number from the left side of the chart to the
right Notice that the element hydrogen is assigned the atomic number of 1.
The atomic number of calcium is _________ .
20
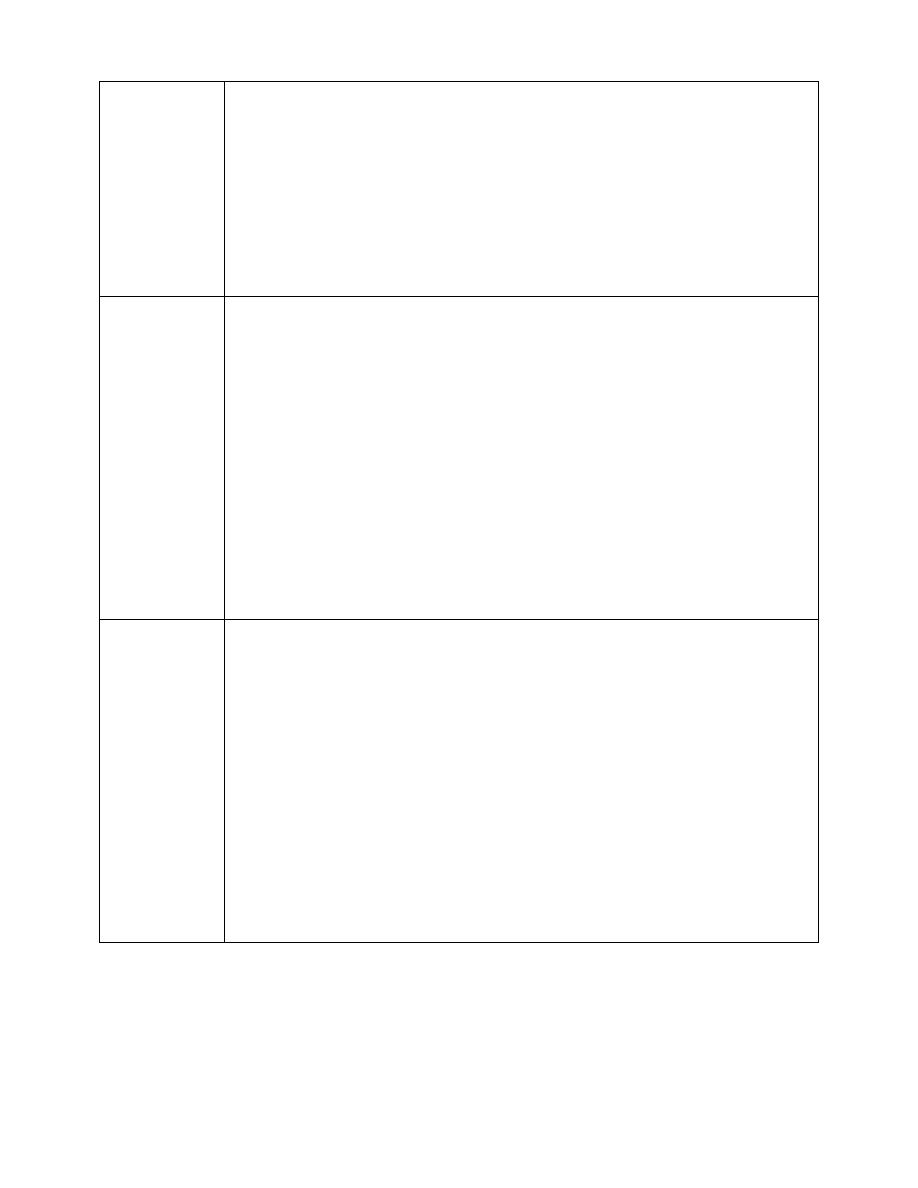
15. Match each term in column A with its definition in column B.
A
B
_____(1) Valence electrons.
a. The outermost subshell.
_____(2) Valence shell.
b. The outermost main shell.
_____(3) Atomic symbol.
c. The abbreviation for each element.
_____(4) Atomic number.
d. The number of protons in the nucleus.
e. The electrons in the outermost shell.
(1) e
16.
State the maximum number of subshells within each main shell of an atom.
(2) b.
(3) c.
(4) d.
IT0341
1-14



 Previous Page
Previous Page
