13.
(Continued)
NOTE: Determination of the number of valence electrons for all elements is
not possible with only the periodic chart. For any element referred
to in this program, however, the number of valence electrons can be
found by using the method described on the pervious page.
The element oxygen is in group VI and period 2; thus, it has six valence
electrons and two electron shells. Carbon has __________________
_______
valence electrons and _____________________ electron shells.
four
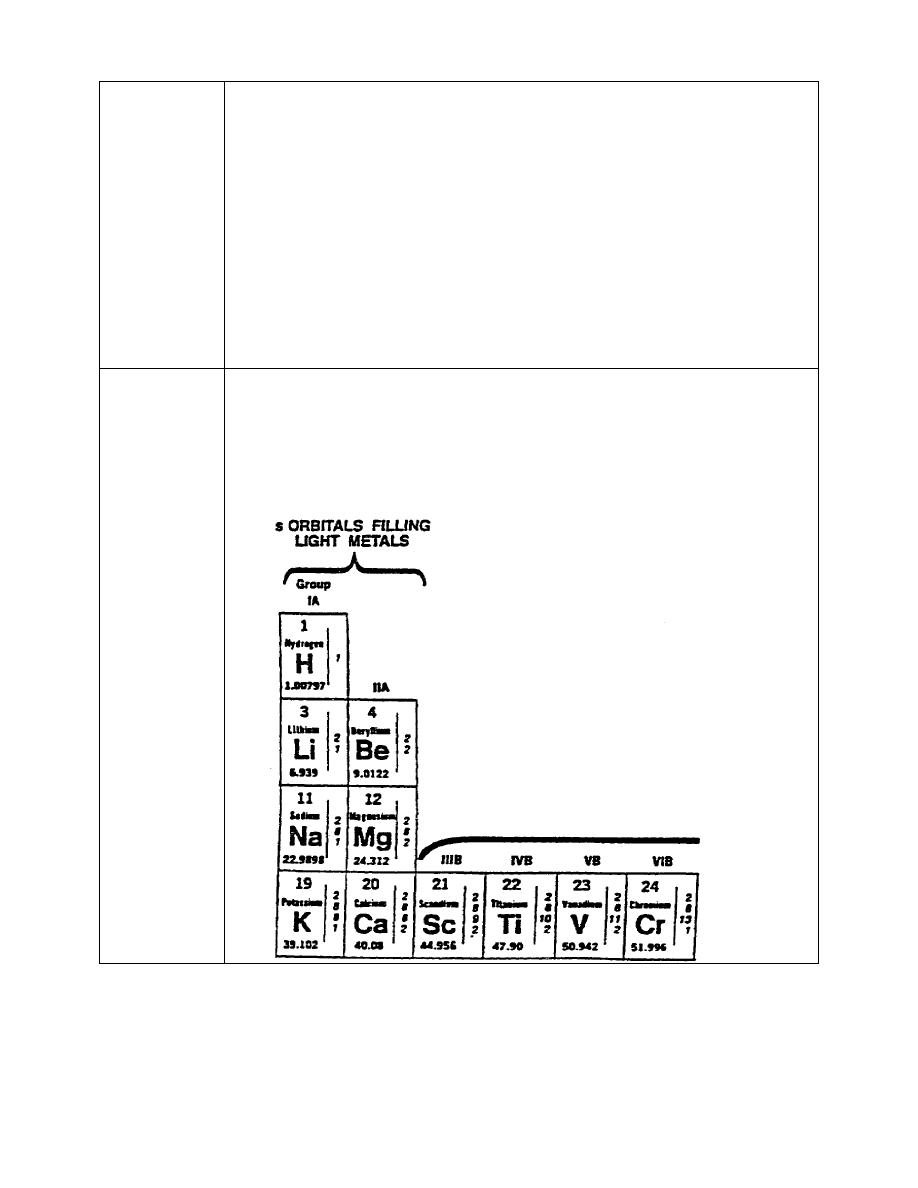
14. All the elements on the periodic chart are arranged in the order of their
two
atomic number, on each block on the chart. As shown in the section of
periodic chart below, the atomic number of magnesium is 12.
1-13
IT0341



 Previous Page
Previous Page
